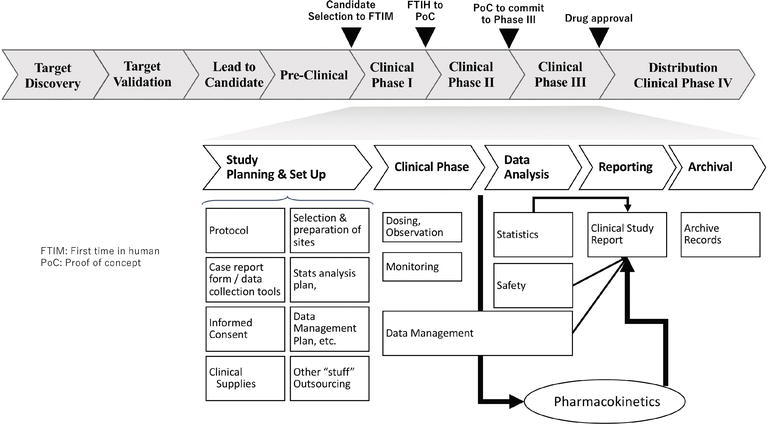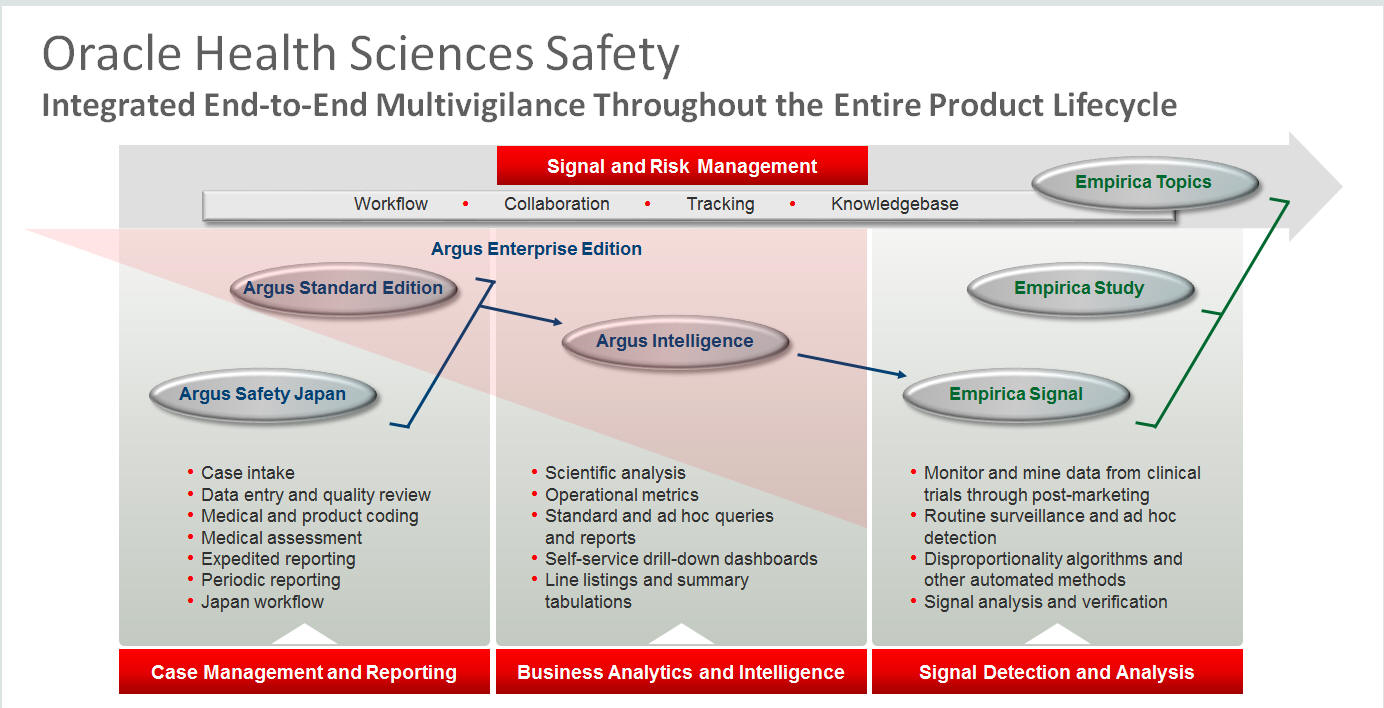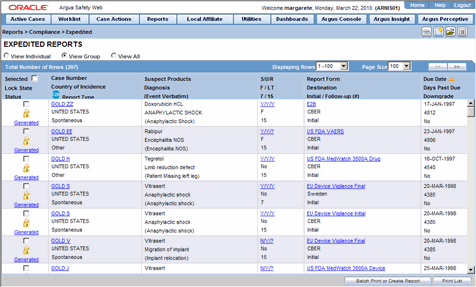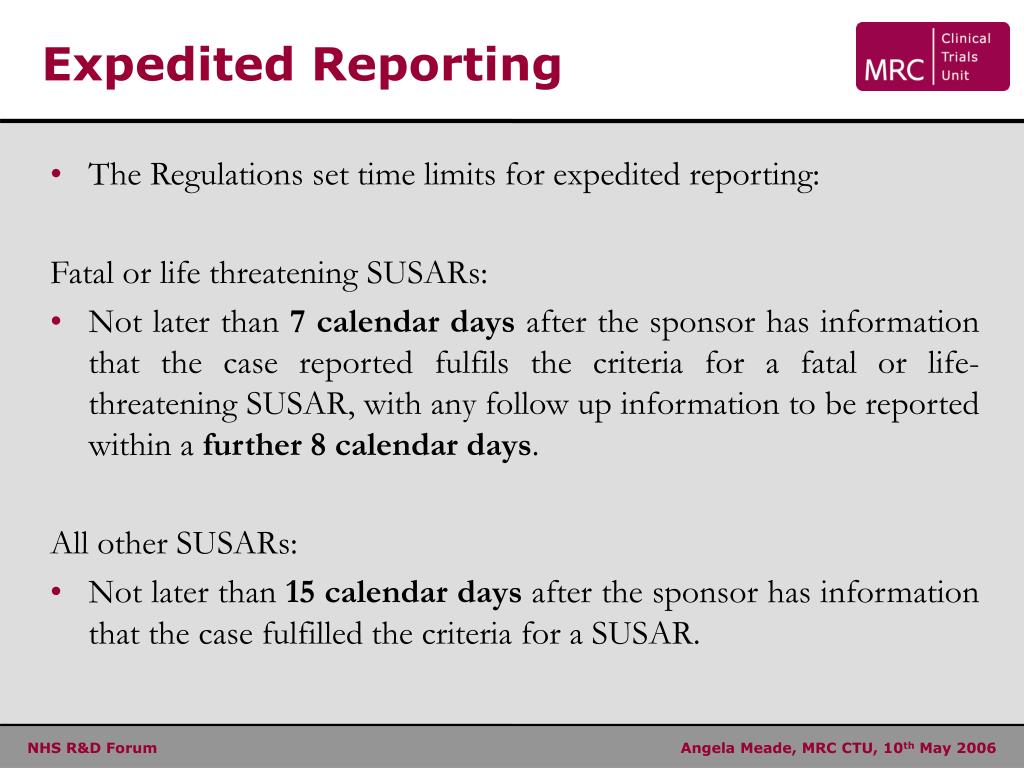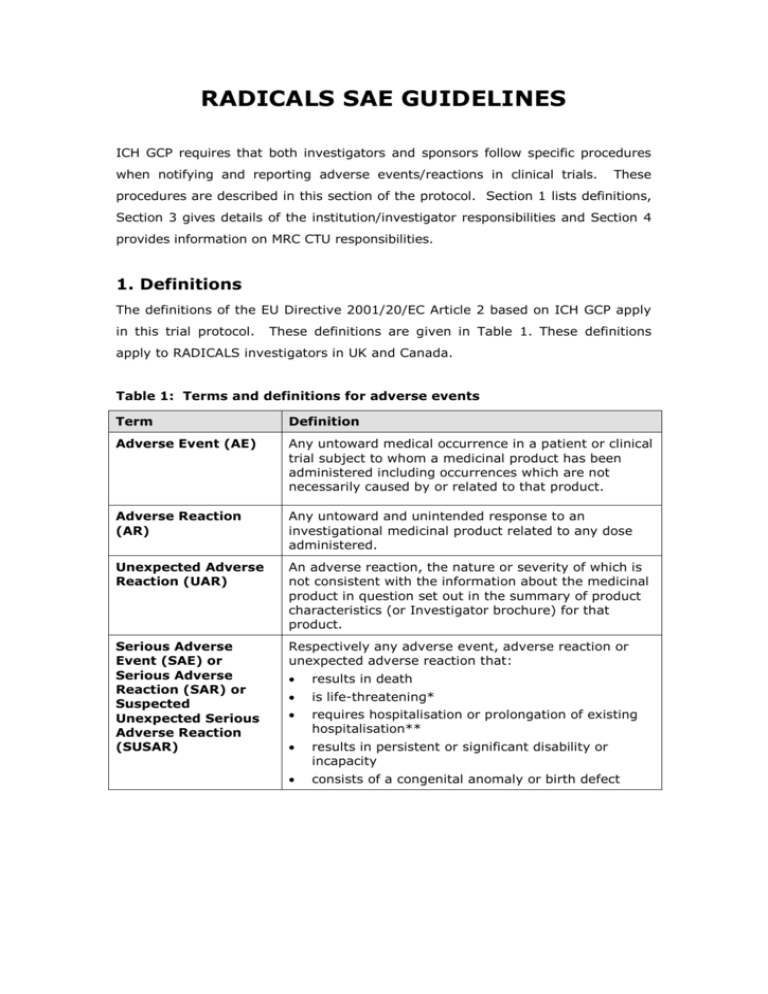
Pharmacovigilance - A Complete Guide to Pharmacovigilance and Drug Safety — Clinical Research Certification

Pharmacovigilance for clinical trials in India: Current practice and areas for reform Brahmachari B, Fernandes M, Bhatt A - Perspect Clin Res

Book 6: 2021 Clinical Trials in The EU: Selected Legislation, Guidelin – Clinical Research Resources, LLC

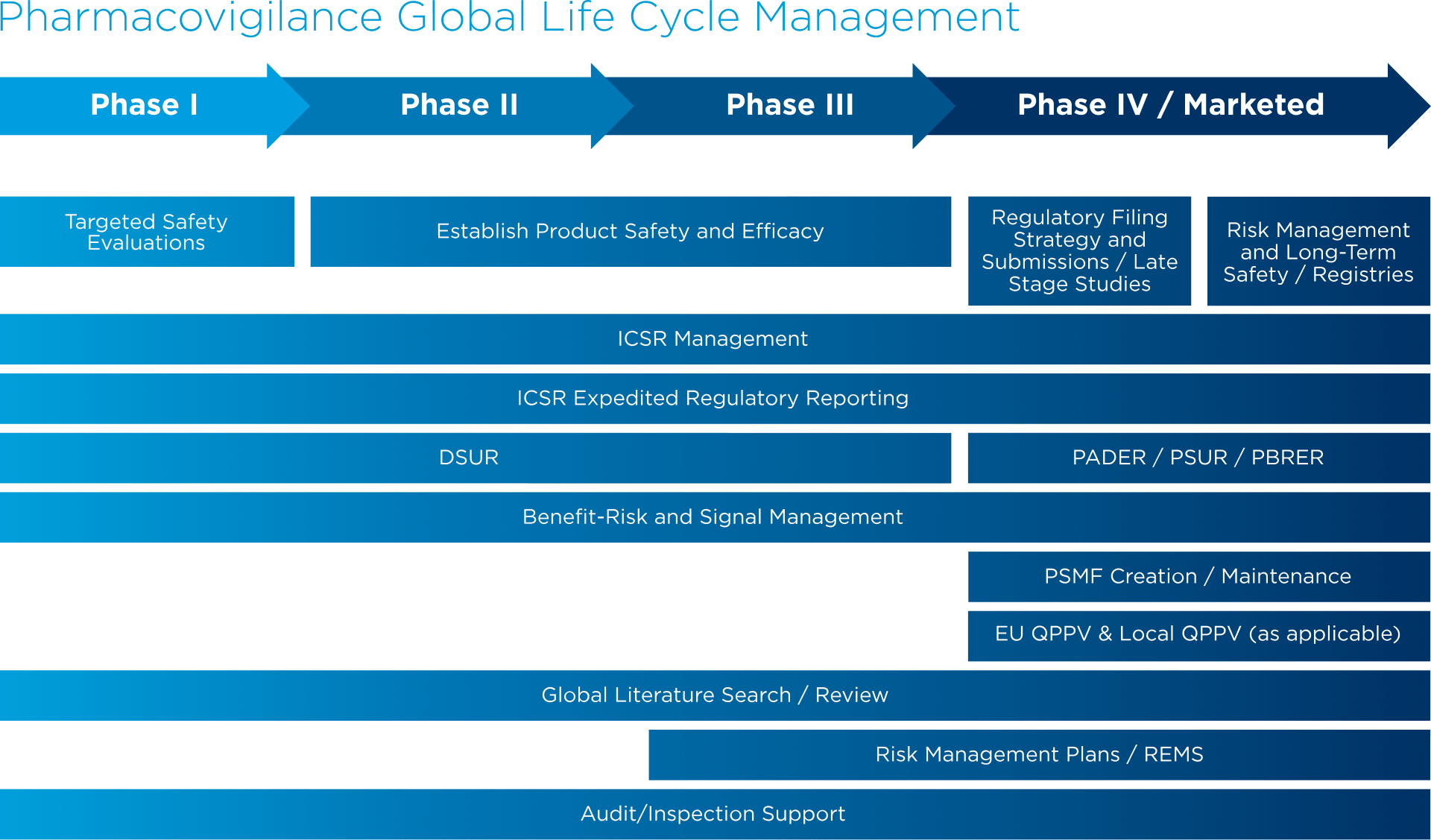
Expedited Reporting-Personnel Communication Flow Chart (Amended: 13 Nov... | Download Scientific Diagram