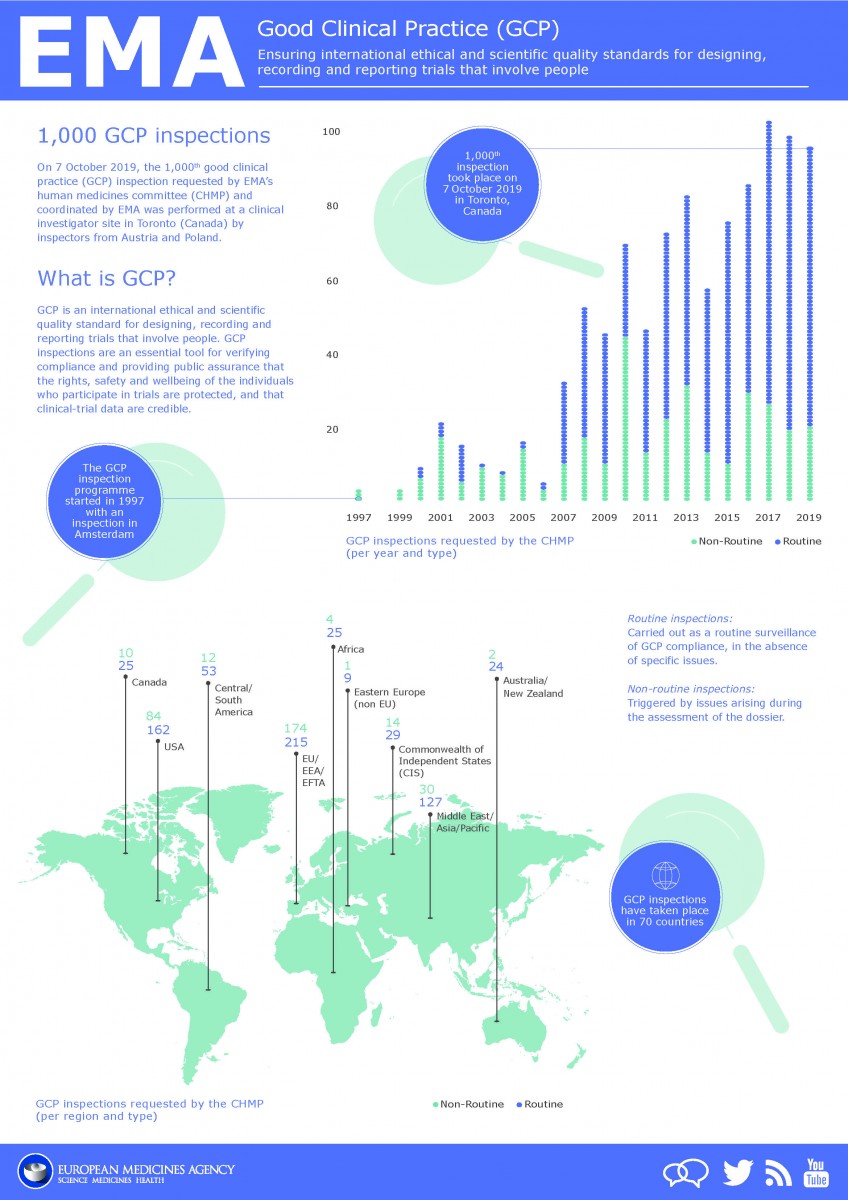
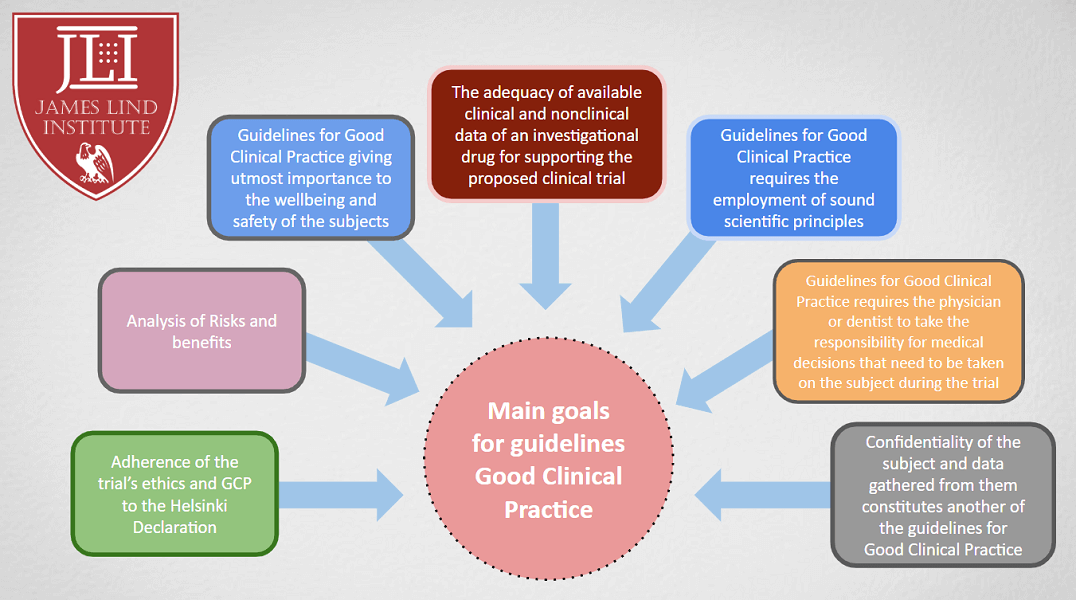
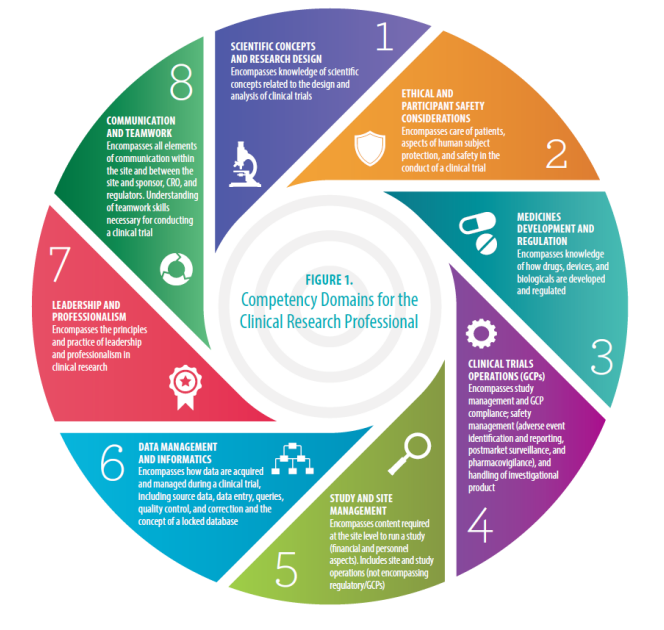
![PDF] The importance of Good Clinical Practice guidelines and its role in clinical trials | Semantic Scholar PDF] The importance of Good Clinical Practice guidelines and its role in clinical trials | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/7a90efbaf9d59ee20353e700230ccbf1660f16b5/3-Table2-1.png)
PDF] The importance of Good Clinical Practice guidelines and its role in clinical trials | Semantic Scholar

Clinical Trials Audit Preparation: A Guide for Good Clinical Practice (GCP) Inspections: Mihajlovic-Madzarevic, Vera: 0000470248858: Books - Amazon

REFRESHER: ICH Good Clinical Practice (GCP) E6 (R2) and regulatory requirements for Clinical Trials (Fiona Stanley Hospital) - RETProgram

The Good Clinical Practice (GCP) and the responsibilities of pharma sponsors - Avantyo article in Viata Medicala magazine · News · Avantyo
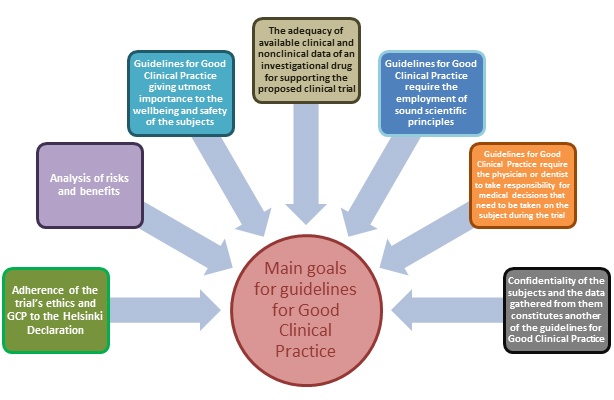
The Good Clinical Practice (GCP) and the responsibilities of pharma sponsors - Avantyo article in Viata Medicala magazine · News · Avantyo

Capacity Building - The Multi-Regional Clinical Trials Center of Harvard and Brigham and Women's Hospital

EU clinical research framework. ICH GCP = International Conference on... | Download Scientific Diagram

ICH Good Clinical Practice (GCP) E6 (R2) and regulatory requirements for Clinical Trials (Curtin University) - RETProgram